 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |

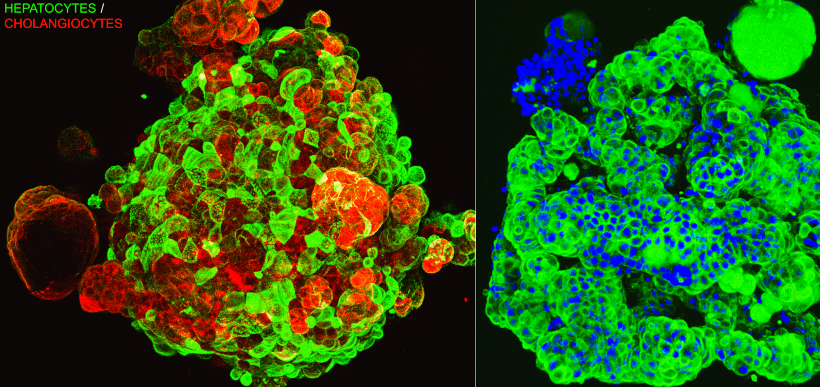
As a complex and vital organ responsible for metabolism and detoxification, the liver is particularly susceptible to toxicity caused by various chemicals, including drugs. Drug-Induced Liver Injury (DILI) is a major cause of drug failures in development. Hepatotoxicity refers to liver damage caused by chemicals, either directly by the drug itself or indirectly through the generation of reactive metabolites. In vitro cell-based toxicity assays offer a valuable approach to mimic in vivo tissue studies and provide a reliable tool for safety evaluation in the early stages of drug discovery. Life Technologies recognizes the importance of assessing drug hepatotoxicity potential during the preclinical stage.
Life Technologies has developed multiplexed hepatotoxicity assays utilizing various in vitro hepatology models, including primary hepatocytes, HepG2 cell lines, and 3D hepatic models. These models are valuable tools for predicting Drug-Induced Liver Injury (DILI). Our experienced scientists are dedicated to assisting you in achieving your objectives and providing support for your projects.
Life Technologies is committed to delivering highly reliable data on drug hepatotoxicity. With our state-of-the-art infrastructure and expert team, we offer comprehensive preclinical research services to assess the safety of drugs and active molecule products. Our customized solutions are designed to meet your specific needs, ensuring enhanced efficacy and safety measures.
Techniques Used: Flow Cytometry, Confocal/Fluorescence Microscopy (Multi-colour Immunofluorescence), ELISA (Fluorimetry/Colorimetry), RTqPCR, WB.
 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |
