 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |

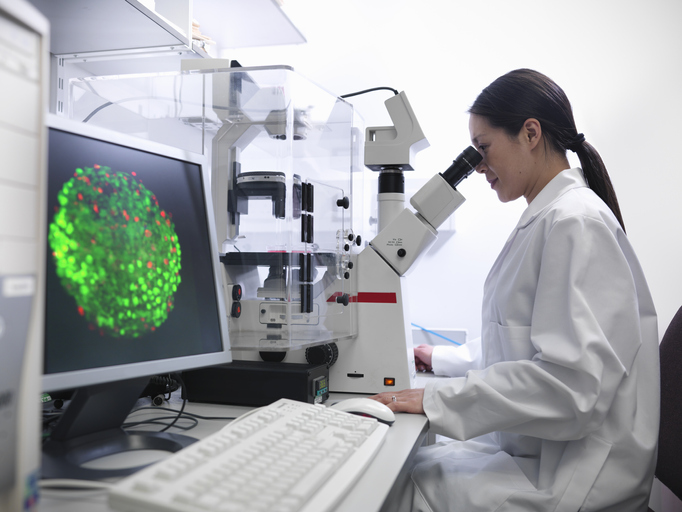
For many investigations that are crucial to determining the safety of the drug, hepatotoxicity is a crucial component of any drug testing method. Because the largest organ is the first to be affected by toxicity during drug development, this has an effect on numerous drug discoveries. We frequently streamline the procedure and permit a few late-stage drugs to participate in in vivo investigations. Time and money will be saved by taking this action. Life Technologies performs tests on human primary cells, however it can be difficult to forecast the drug-induced liver injury because it can be caused by a number of different things. The disruption of mitochondrial activity and inflammatory reactions, as well as reactive metabolites and reactive oxygen species (ROS), can affect the liver's defensive mechanisms and cause cellular damage. As a result, we evaluate the DILI circumstances using a variety of tests.
Life Technologies experience has been utilised by us to conduct two panels of tests that call for a high-throughput screening panel. We will be able to quickly and clearly evaluate the hepatoxicity.
A panel of 8 assays measuring 12 endpoints provides a thorough evaluation of known indicators of DILI, such as human hepatocyte toxicity, mitochondrial dysfunction, the production of reactive metabolites, cholestasis, and phospholipidosis, as well as important cellular markers of liver injury, such as cell viability, nuclear size, mitochondrial membrane potential, intracellular free calcium, and cell membrane integrity.
 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |
