 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |

New drug development is a time-consuming and high-cost process. Drug repurposing (also called drug repositioning, reprofiling or retasking) offers various advantages over developing an entirely new drug for a given indication. First, the risk of failure is lower. Second, the time frame for drug development can be reduced. Third, less investment is needed. Approved drugs have identified bioactivities, good pharmacokinetic characteristics and safety which are suitable for drug repurposing.
MCE owns a unique collection of 2710 approved compounds which have been completed extensive preclinical and clinical studies and have well-characterized bioactivities, safety and bioavailability properties. MCE FDA-Approved Drug Library is a good tool for drug repurposing which could dramatically accelerate drug development.
MCE FDA-Approved Drug Library can be supplied as pre-dissolved Solutions or Solid. For pre-dissolved solutions in this library, there are 2623 compounds supplied in 10 mM solution, 64 compounds supplied in 2 mM solution and 23 compounds supplied in 3 mg/mL solution.
For pre-dissolved solutions, 10 mM for compounds with the solubility not lower than 10 mM, 2 mM for compounds with solubility between 2 mM and 10 mM, and 3 mg/mL for compounds with unconfirmed molecular weight and solubility not lower than 3 mg/mL.

Size (Pre-dissolved Solution or Solid)
Publications Citing Use of MCE Compound Libraries
• Nat Microbiol. 2023 Jan;8(1):121-134. [Abstract]
• Nat Commun. 2020 Nov 12;11(1):5722. [Abstract]
• Nat Commun. 2018 Dec 10;9(1):5272. [Abstract]
• Int J Antimicrob Agents. 2019 Oct;54(4):502-506. [Abstract]
• Sci Adv. 2019 May 22;5(5):eaav6528. [Abstract]
• Sci Adv. 2021 Dec 24;7(52):eabb3673. [Abstract]
• Pharmacol Ther. 2021 Jun 23;228:107930. [Abstract]
• Cancer Immunol Res. 2023 Mar 15;CIR-22-0483. [Abstract]
• Theranostics. 2018 Jan 1;8(3):830-845. [Abstract]
• Environ Sci Technol. 2021 Jan 20. [Abstract]
• EBioMedicine. 2022 Dec 8;87:104397. [Abstract]
• Cell Chem Biol. 2021 Sep 8;S2451-9456(21)00400-1. [Abstract]
• J Cell Biol. 2023 Jan 2;222(1):e202202110. [Abstract]
• PLoS Pathog. 2021 Sep 3;17(9):e1009898. [Abstract]
• Commun Biol. 2021 Feb 15;4(1):203. [Abstract]
• Int J Mol Sci. 2022 Apr 28;23(9):4891. [Abstract]
• Viruses. 2019 Apr 25;11(4):385. [Abstract]
• J Antimicrob Chemother. 2022 Nov 2;dkac370. [Abstract]
• ACS Infect Dis. 2021 May 21. [Abstract]
• J Biomol Struct Dyn. 2021 Jan 7;1-17. [Abstract]
• Heliyon. 2020 Aug;6(8):e04793. [Abstract]
• Med Mycol. 20 September 2022.
• SLAS Discov. 2023 Apr 28;S2472-5552(23)00034-5. [Abstract]
| Description & Advantages | |||||||||||||||||||
|
• A unique collection of 2710 approved drugs for high throughput screening (HTS) and high content screening (HCS). • Used in the research of oncology, cardiology, anti-inflammatory, immunology, dermatology, endocrinology, neurology, and more. • A useful tool for researching new targets of marketed old drugs. • All compounds have been approved by the FDA, EMA and other countries. • Structurally diverse, bioactive, and cell permeable. • More detailed compound information with structure, IC50, and brief introduction. • NMR and HPLC validated ensure high purity. • All compounds are in stock and continuously updated.
|
|||||||||||||||||||
| Product Details | ||||||
|
• Formulation:A collection of 2710 marketed drugs supplied as pre-dissolved Solutions or Solid • Layout: 96-well storage tube or 96-well plate: 1st and 12th column are left empty. 384-well plate: the first two columns and the last two columns are left empty. Compounds with different concentrations or dissolved in different solvents will be put on separate plates. This way of layout may increase the number of plates because there could be three solvents and three concentrations. If you have other requirements, please let us know. • Container:96- or 384-well Plate with Peelable Foil Seal; 96-well Format Sample Storage Tube With Screw Cap and Optional 2D Barcode • Storage:-80°C • Shipping:Blue ice |
||||||
| Composition | ||||||
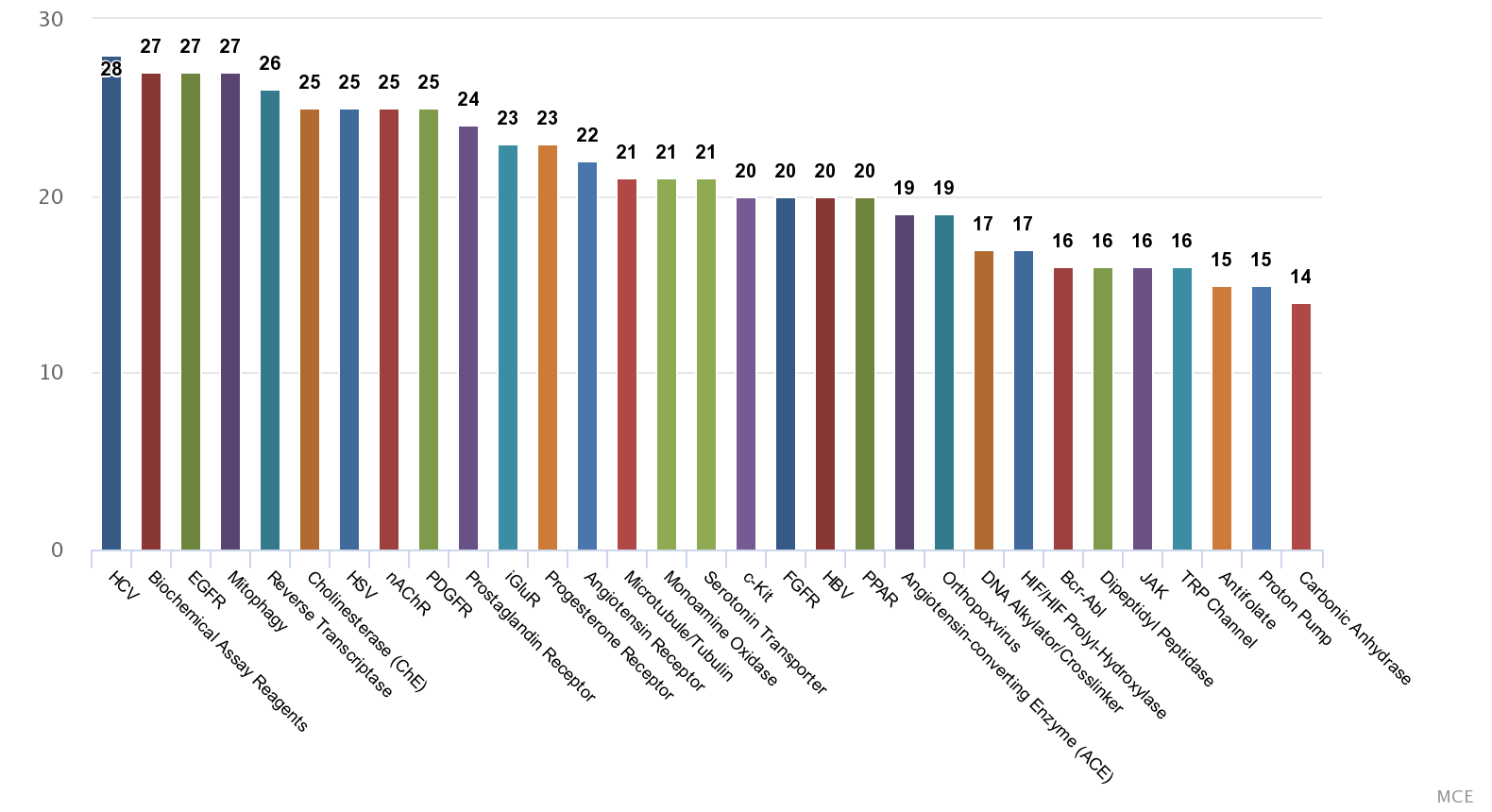
| Natural Product Library (Source: MedChem Express) | ||||||
|---|---|---|---|---|---|---|

|
||||||
|
||||||

|
||||||
| Documentation | ||||||
 |
Life Technologies (India) Pvt Ltd. 306, Agarwal City Mall, opposite M2K Pitampura, Delhi-110034 (India) Tel # +91-11-4220-8000; 4220-8111; 4220-8222 Fax# +91-11-4220-8444, Mobile# +91-98105-21400 Email# customerservice@lifetechindia.com |
